Supercapacitor: Myths vs Reality
As new technology is constantly entering and leaving the market, interpretation and understanding tends to come with some misbeliefs. Let’s clear up some of the more common myths and open the topic up for learning and discussion!
Myth: Capacitors, Supercapacitors and Batteries do the same thing.
Reference: exponent.com
Reality: Capacitors, supercapacitors and batteries all store energy. The difference is how much energy they can store, as shown in their specific energy (Wh/L) or energy density (Wh/Kg) ratings. They also differ in the speed or rate in which their energy can be delivered, as shown in their specific power (W/L) or power density (W/Kg) ratings. Batteries store more energy, but capacitors store more power. These differences in performance are from each technology’s difference in materials, chemistry, and construction.
Capacitors have two conductive plates separated by a non-conductive layer, often called a dielectric. This gives the capacitor the possibility of charging/discharging to a high voltage if the non-conductive dielectric layer is thick enough. The main energy storage mechanism in capacitors is from an electric field between the two conductive layers.
Batteries also consist of two conductive layers, but between there is an electrolyte that helps create chemical reaction between those layers. The electrolyte may be a liquid chemistry that is absorbed by a separator placed between the plates, or may be a solid electrolyte (which acts as the separator) between the plates. While there is still an electric field, the conductive layers are much further away from one another than in a capacitor making the electric field weak. The chemical reactions are the main energy storage mechanism of batteries. The chemical reactions take time, but is what allows for a much higher energy density. Also, chemical reactions happen at very specific voltages, so batteries are limited in voltage by the underlying chemical reaction.
Supercapacitors, also known as ultracapacitors or electrochemical double layer capacitors, combine the structure of capacitors and batteries. Just like with capacitors and batteries, supercapacitors have two conductive layers. Like a traditional capacitor and unlike a battery, the conductive layers in a supercapacitor are very close to one another helping it to leverage an electric field as an energy storage mechanism. However, between the plates, the supercapacitor looks similar to a battery in terms of having a liquid chemistry absorbed by a separator between the plates, or using a solid electrolyte between the plates. Supercapacitors can therefore take advantage of two energy storage mechanisms, the electric field found in a capacitor and the chemical reactions found in a battery.
Our Cable-Based Capacitors (CBC’s) are unique in that the structure consists of flexible layers in a coaxial cable form factor instead of the traditional rigid layers.
It should be noted that different materials and chemistries are used in capacitors, batteries, and supercapacitors, which optimize their performance based on their intended applications.
Myth: Supercapacitors can be charged and discharged like a battery.
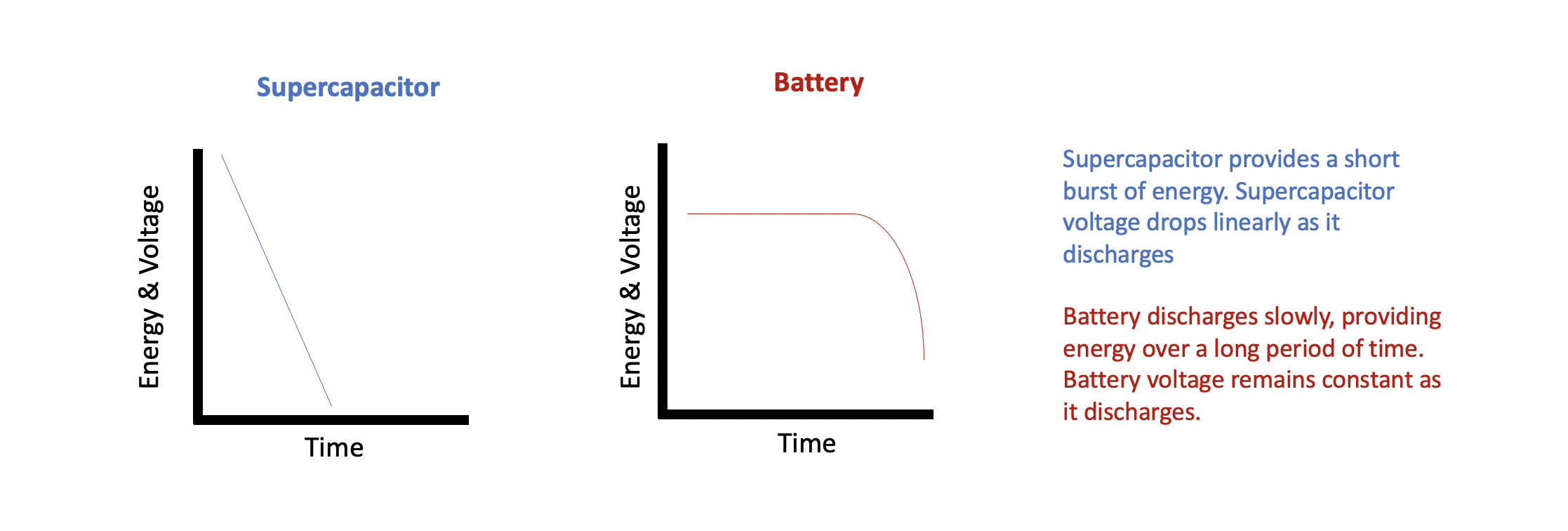
Reality: As mentioned above, batteries store electrical energy through chemical reactions. These chemical reactions happen at a specific voltage. Thus, batteries have a relatively small voltage band between being fully charged and discharged. This can make it somewhat tricky to actually gauge how much charge is left. The battery’s service life is influenced by temperature, charge/discharge rate, and number of previously charge/discharge cycles. A battery will need time to reach a full charge, but is then able to discharge at a constant rate over a long period of time.
Supercapacitors are more like electrical charge sponges. They will soak up charge at a constant high rate until full. This makes them constant charge devices, unlike the nearly constant voltage characteristic of batteries. This also allows for rapid charge and discharge of electrical energy. While unable to discharge over a long period of time, the ability to rapidly change charge status allows for supercapacitors to endure spikes of electrical energy without damage as well as smoothing out any fluctuations and dispersing any detrimental noise.
Supercapacitors, including our CBC’s, depend on a very thin layer to keep charged ions separate. This limits the maximum voltage when the thin layer breaks down as the voltage gets too high.
Myth: Supercapacitors last forever.
Reality: This is more of a common misconception rather than a myth. Supercapacitors are based on a structure that does not wear out as easily as deep-cycle batteries. Where batteries are often limited to a pretty standard number of charge/discharge cycles, supercapacitors have a near infinite number of charge/discharge cycles, giving the idea that they last forever. Unfortunately, nothing lasts forever. As mentioned, supercapacitors are damaged when voltage surpasses the maximum limit for a device for an extended period of time. High and sub-freezing temperatures may have a similar effect. Our CBC’s do work at a lower temperature than batteries, but neither batteries nor CBC’s function well with excessive voltage, high, or sub-freezing temperatures. Respect the specified limitations of a technology and be aware of those varying limitations for your chosen device. Supercapacitors have a much higher number of cycles than batteries do, and therefore have a longer service life. We talked about that in a previous blog post. They are more rugged and able to handle fluctuations in power better than batteries, but they do not last forever.
Myth: Supercapacitors store as much energy per volume as batteries.
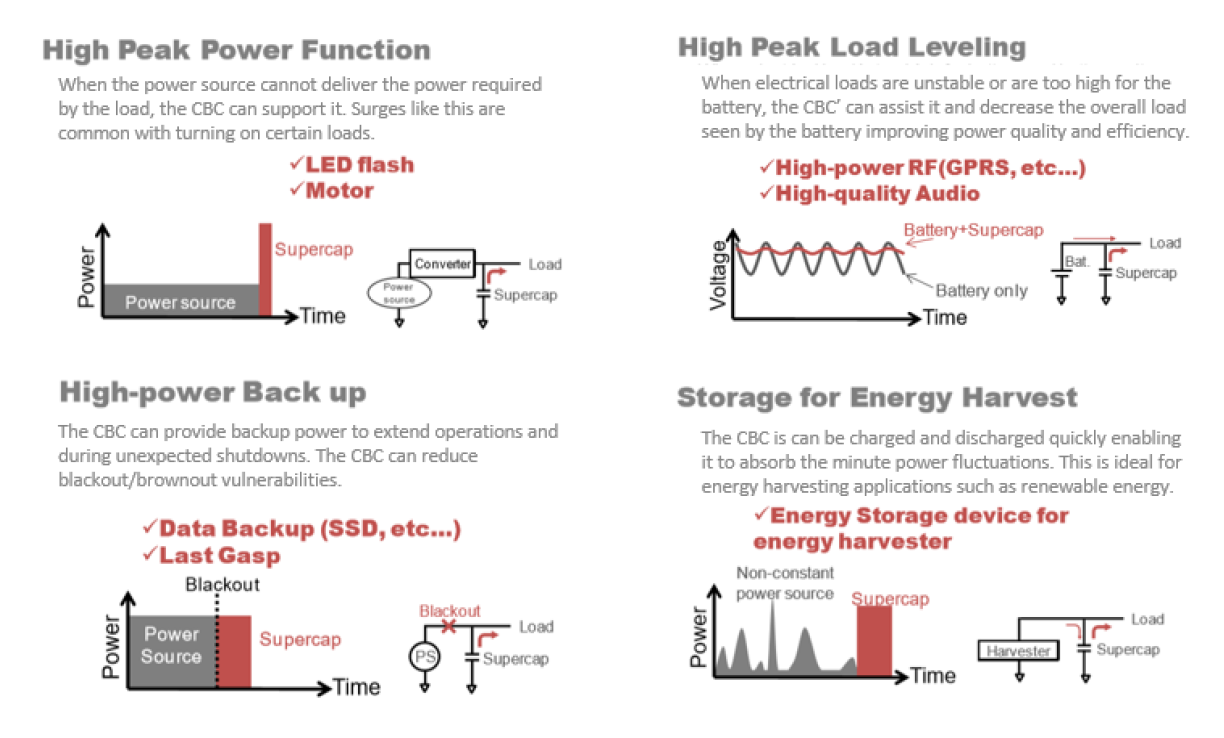
Reality: The mechanism of storing electrical energy in supercapacitors through ions does not have anywhere near the energy density of batteries. In fact, as it stands, batteries can store anywhere from 10 to 100 times the amount of energy density that supercapacitors are able. However, this misses the point of using supercapacitors and CBC’s for their original and intended purpose. CBC’s can accept and deliver their electrical energy much faster and more efficiently than batteries can due to their large power density. Ideal uses of CBC’s are summarized in this illustration.
Myth: Supercapacitors can be used as a substitute for ceramic or bipolar capacitor.
Reality: CBC’s are not designed for alternating current (AC). They are direct current (DC) only devices. They are great at smoothing out voltage fluctuations as long as the current, and in turn, voltage, does not reverse in direction.
Myth: Short circuiting supercapacitors damages them.
Reality: Short circuiting batteries causes heating that will ruin them for a multitude of reasons from melting cell separators, to changing chemistry, to massive discharges and draining of the cell(s). As mentioned, excessive heat is not ideal for electrical energy storage devices. However, CBC’s have a much lower internal resistance. This means that they are much less likely to experience internal heating, therefore preventing any damage from occurring due to a short circuit. The heat generated by a short circuit is much more likely to heat the wire causing the “short” or weld the contacting metals causing the short together. Connecting the two leads of the CBC will quickly discharge the supercapacitor.
Myth: Supercapacitors require lots of room in a design.
Reality: While this is true for competing supercapacitors, our CBC’s are different. The thin, wire-like design allows for flexibility far beyond the capabilities of the rigid “beer can” capacitors, allowing the CBC’s to be used in mechanical arrangements and wiring infrastructure where no other supercapacitors will fit. More simply stated, the body of traditional supercapacitors take up real estate or surface area on the printed circuit board, but the CBC’s body is lifted off the board so that the space can be used for an different purpose (added features or miniaturization). For example, they can become part of the power supply harness, be bent and formed in ways that work within the structure of a device, or even miniaturize a product by attaching around a circuit rather than forced to be placed on the face.
Take a look at this video where the CBC stores the energy from a solar cell right in the wiring. Revolutionary, wouldn’t you agree? The CBC is great for applications that need a lot of energy delivered quickly (burst or pulse power). Our form factor helps designers save space.
Which of these myths did you believe? Now that we’ve gotten some misunderstandings out of the way, what others have you potentially heard? Let us know any additional questions or thoughts you may have below and let’s start a conversation!